Siemens Advia Centaur XPT Immunoassay System
In Stock
Product Code: SCXPT001
Manufacturer: Siemens Healthineers

Meet your sales rep Lee Doughton, for the West USA region.
Would you like to schedule a 30-minute consultation with Lee Doughton?
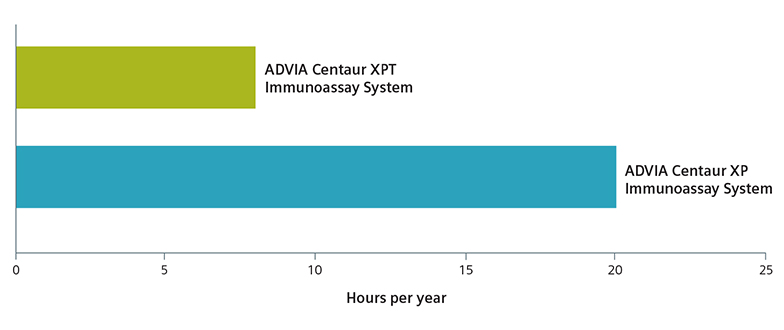
Yes, Schedule a callDesigned with one of the most advanced software packages on the market, the ADVIA Centaur® XPT System is among the highest-throughput systems available. The ADVIA Centaur XPT System delivers the results that clinicians depend upon for accurate diagnoses and better patient care—and does so predictably and consistently.
Siemens unites innovative workflow solutions with clinical excellence in the ADVIA Centaur XPT System, leading to greater laboratory productivity to stay ahead of increasing workload demands.
- Simplified operation enhances lab efficiency.
- Cutting-edge immunoassay testing supports clinicians in the screening, diagnosis, and monitoring of the most complex diseases.
- Powerful automation and software streamline and accelerate your workflow.
Features & Benefits
Simplified, continuous operation
- Intuitive, icon-driven user interface for simplified training and operation
- On-the-fly loading and unloading of samples, reagents, and supplies
- 2D bar-code scanner uploads test definitions with one easy scan, saving valuable time
- Automated daily maintenance and no monthly cleaning procedures
- No daily startup procedure
- Status light displays system alerts visible from anywhere in the laboratory
Accurate, timely results
- Comprehensive menu supports over 70 tests*
- Versatile, advanced AE technology, with over 40 active patents, enables continual introduction of new and innovative assays
- Up to 240 tests per hour for fast turnaround, even during peak workload times
- STAT port allows priority sampling at any time, even when connected to automation
- Highly stable, ready-to-use reagents are used on all ADVIA Centaur systems
- Disposable sample tips ensure no sample-to-sample carryover
- Automated clot management virtually eliminates operator intervention
- SMART algorithm software automates repeat and confirmatory testing of reactive samples
Powerful, seamless connectivity
- Designed for automation; requires no additional robotics
- Point-in-space technology enables direct-from-track sampling
- STAT samples can be front-loaded at any time for immediate sampling, driving rapid turnaround time (TAT)
- Direct connectivity to Siemens Automation Solutions, the CentraLink™ Data Management System, and Siemens Remote Service (SRS) , enabling laboratories to adapt and grow as their workloads demand
Anemia
Active B12
Ferritin
Folate
RBC Folate
Vitamin B12
Autoimmune
Anti-CCP
Bone Metabolism
Intact PTH
Vitamin D Total
Cardiac
BNP
CKMB
High-Sensitivity Troponin I (TNIH)
Myoglobin
TnI-Ultra™
Diabetes
C-Peptide
Insulin
Liver Disease – NASH
ELF™ [Enhanced Liver Fibrosis]
Hepatitis
Anti-HBs2
HAV IgM
HAV Total
HBc IgM
HBc Total
HBe Ag
HBs Ag Confirmatory
HBs AgII
HCV***
HIV
HIV 1/O/2 Enhanced (EHIV)§
HIV Combo§
Immunosuppressant Drugs
Cyclosporine
Everolimus**
Tacrolimus**
Inflammation
IgE, Total
Metabolic
Cortisol
Homocysteine
Oncology
AFP
BR 27.29
CA 125II‡
CA 15-3
CA 19-9
Calcitonin
CEA
Complexed PSA
PSA
Serum HER-2/neu
Reproductive Endocrinology
AFP
Anti-Müllerian Hormone**
DHEAS
Enhanced Estradiol
Free βhCG‡‡
FSH
hCG
LH
PAPP-A‡‡
PlGF**
Progesterone
Prolactin
sFLT-1**
SHBG
Testosterone II
Special ID
EBV-EBNA IgG**
EBV-VCA IgG**
EBV-VCA IgM**
Syphilis
Zika IgM††
Therapeutic Drug Monitoring (TDM)
Carbamazepine
Digitoxin
Digoxin
Gentamicin
Phenobarbital
Phenytoin
Theophylline
Valproic Acid
Vancomycin
Thyroid
Anti-TG
Anti-TPO
Free T3
Free T4
T Uptake
Total T3
Total T4
TSH3-Ultra
TSH
ToRCH
CMV IgG
CMV IgM**
Herpes-1 IgG
Herpes-2 IgG
Rubella IgG
Rubella IgM
Toxoplasma IgG
Toxoplasma IgM
Technical Specifications
| Product Specifications | |
|---|---|
| System Description | Random-access immunoassay system |
| Test Throughput | Up to 240 tests per hour |
| Time to First Result | 18 minutes; results every 15 seconds thereafter, assay dependent |
| Assays Onboard | 30 assays |
| Disease-State Assay Groups | Allergy, Anemia/Iron Metabolism, Bone Metabolism, Cardiac, Diabetes, Hepatitis, Immunosuppressant Drugs, Metabolic, Oncology, Reproductive Endocrinology, Special ID, Therapeutic Drug Monitoring (TDM), Thyroid, ToRCH |
| Continuous Operation | Loading/unloading of samples and consumables at any time without interrupting system operation. Includes sample racks, STATs, reagent packs, ancillary reagent packs, reaction cuvettes, sample pipette tips, calibrators, controls, wash fluids, water, and waste. |
| Sample Handling | |
| Sample Tubes | 3 mL, 5 mL, 7 mL, and 10 mL tubes; 1 mL and 2 mL sample cups; microcontainer tubes |
| Sample Input | Universal 5-position rack holds multiple tube types; 180 samples fully loaded; no-pause loading and unloading |
| Validated Sample Types | Serum, plasma, urine (assay-dependent) |
| Sample Volume per Test | 10–200 μL, assay-dependent |
| Sample Integrity Control | Pressure transducer technology with clot detection, flagging, and management; short-sample detection |
| STAT Handling | Dedicated STAT port accepts samples any time; STAT samples are processed with priority |
| Auto-repeat | User-definable |
| Sample Dilution | Automatic dilution varies by assay, up to 1:2500 |
| Auto-reflex Testing | Automatic ability to perform additional tests based on results of first test |
| Sample Pipette Tips | 840 disposable sample tip capacity |
| Sample Carryover Prevention | Disposable pipette tips ensure zero sample carryover |
| Bar Codes | |
| Sample Bar Codes | Up to 20 digits; Interleaved 2 of 5; Code 39, Code 128, Codabar; A, B, and special characters (.-+/*$%) |
| Reaction Area | |
| Reaction Cuvettes | Capacity of 1000 optical-grade plastic cuvettes |
| Reaction Bath | Air bath, 37°C |
| Assay Result Calculations | SMART algorithm software repeats and confirms reactive testing; results available as completed |
| Assay Technology | Chemiluminescence using Advanced Acridinium Ester |
| Reagent Handling | |
| Reagent Tray | 30-position reagent tray refrigerated between 4 and 10°C (39–50°F) |
| Reagent Capacity Onboard | 30 assays |
| Reagent Containers | ADVIA Centaur® ReadyPack® (various pack sizes available) |
| Reagent Integrity Control | Bar-code reagent identification, automatic inventory tracking and flagging, calibration valid tracking and flagging, reagent onboard stability tracking and flagging, reagent expired/reagent low flagging |
| Onboard Stability | 28 days (average) |
| Reagent Mixing | ADVIA Centaur ReadyPacks automatically rocked onboard |
| Ancillary Reagents | 25-position ancillary reagent tray cooled 4–8°C (39–46°F) |
| Calibration/QC | |
| Auto-calibration/Auto-QC | Available automatic QC ordering by test, control, date, and time |
| QC Data | Advanced QC package includes Levey-Jennings plots and Westgard rules |
| User Interface/Data Management | |
| Monitor | 22-inch diagonal high-resolution LCD touchscreen with adjustable height |
| Operating System | Microsoft® Windows® 7 |
| System Documentation | Operator manual, quickstart guide, and context-sensitive online help |
| Data Storage | 1,000,000 test/QC results; 500,000 can be historical; can archive to removable media |
| Onboard Maintenance Logs | Yes |
| Host Interface | RS232C bidirectional and host query; Ethernet connection available |
| Host Query | Yes |
| Remote Access & Service | 1000Base-T Ethernet port for remote access and modem for Siemens Remote Service |
| Removable Media | |
| Removable Media | CD, DVD, or USB |
| General Specifications | |
| Power Requirements | 200-240 V at 50/60 Hz; 1.2 kW maximum |
| Maximum Water Consumption | 2.5 liters (0.6 gallons) per hour |
| Drain Requirements | Minimum of 2.5 liters (0.6 gallons) per hour |
| Dimensions | With monitor: 167 (h) x 196 (w) x 104 (d) cm; 66 (h) x 77 (w) x 41 (d) in. Without monitor: 133 (h) x 196 (w) x 104 (d) cm; 52 (h) x 77 (w) x 41 (d) in. |
| Weight | 585 kg (1289 lb) |
| Compliance | Complies with international environmental, health, and safety standards |
| Noise Emission | 65 dB maximum |
| Processing Heat Output | 5100 BTU/hour |
| Ambient Temperature | 18–30°C (64–86°F) |
| Ambient Humidity | 20–80% noncondensing |
| Floor Load-Bearing Requirement | 412 kg/m2 (84 lb/ft2) |