For Business Use Only. Does Not Ship to Residential Addresses. For use inside an Analyzer, Sold Separately.
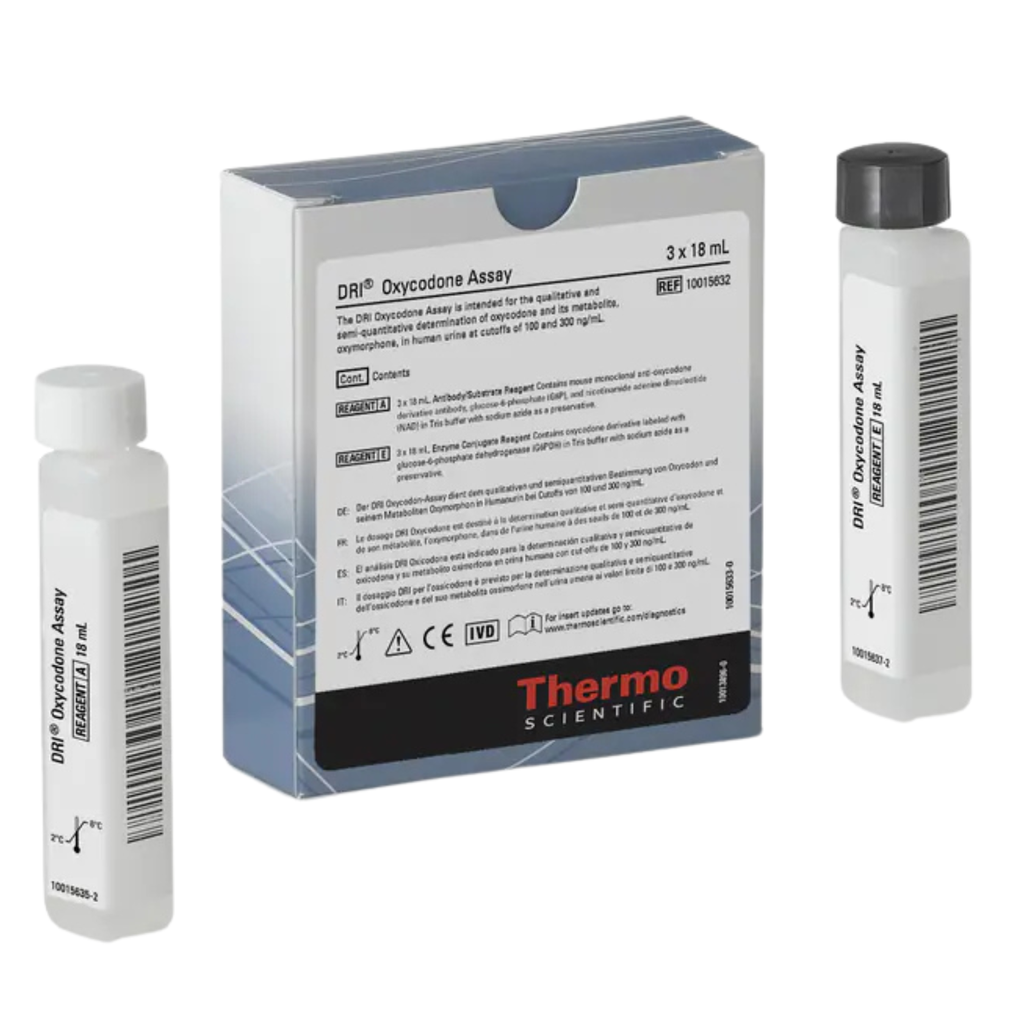
Thermo Kit Oxycodone Rgt DRI
In Stock
Product Code: T-10015632
Manufacturer: Thermo Scientific
Shipping Weight: 2.00lbs (0.91kg)

Meet your sales rep Lee Doughton, for the West USA region.
Would you like to schedule a 30-minute consultation with Lee Doughton?
Yes, Schedule a callThermo Kit Oxycodone Rgt DRI
Thermo Kit Oxycodone Rgt DRI
Specifications
- Control Sets: DRI Oxycodone Control Set
- Description: DRI Oxycodone Drugs of Abuse Assays
- Detectable Analytes: Oxycodone
- DoA Calibrators: DRI Oxycodone Calibrators
- Quantity: 3 x 18mL
- Storage Requirements: 2° to 8°C
Intended Use
The DRI® Oxycodone Assay is intended for the qualitative and semi-quantitative determination of oxycodone in human urine at cutoffs of 100 and 300 ng/mL. The assay provides a simple and rapid analytical screening procedure to detect oxycodone in human urine.
Summary and Explanation of the Test
Oxycodone is a semi-synthetic opioid prescribed for pain management in patients with moderate to severe pain. It is similar to codeine and morphine in its analgesic properties but it is more potent than morphine and has higher dependence potential. The drug oxycodone is supplied as OxyContin® (Oxycodone HCl) or in combination with aspirin (Percodan®) or acetaminophen (Percocet®). Drug abusers crush the pills into powder and snort them for faster effect which may result in a potentially fatal outcome. According to Drug Abuse Warning Network (DAWN), there has been a dramatic increase in oxycodone related deaths. Oxymorphone, noroxycodone and noroxymorphone are the only known metabolites of
oxycodone. The metabolite, oxymorphone, is a potent narcotic analgesic, while the other two metabolites are relatively inactive. From 33-61% of a single dose of oxycodone is excreted in urine within 24 hours as unconjugated oxycodone (13-19%), conjugated oxycodone (7-29%), and conjugated oxymorphone (13-14%).
The DRI Oxycodone Assay is supplied as a liquid ready-to-use homogeneous enzyme immunoassay. The assay uses specific antibodies that can detect oxycodone and oxymorphone without any significant cross-reactivity to other opiate compounds. The assay is based on competition between a drug labeled with glucose-6-phosphate dehydrogenase (G6PDH), and free drug from the urine sample for a fixed amount of specific antibody binding sites. In the absence of free drug from the sample, the specific antibody binds the drug labeled with G6PDH and causes a decrease in enzyme activity. This phenomenon creates a direct relationship between the drug concentration in urine and enzyme activity. The enzyme activity is determined spectrophotometrically at 340 nm by measuring the conversion of nicotinamide adenine dinucleotide (NAD) to NADH.